CareStart™
COVID-19 Antigen Home Test
Self Test for SARS-CoV-2 Antigen Detection
$21.99 + Shipping for TWO Tests
01.
Convenient
Fast and easy to self-test anywhere
02.
Easy to Understand
Easy to interpret the results using mobile application
03.
Clear
Qualitatively detect the SARS-CoV-2 nucleocapsid protein
04.
Accessible
Use for nasal swab specimen
05.
Efficient
Fast results only in 10 minutes
06.
Accurate
Identify individual’s current infection status to COVID-19
The CareStart™ COVID-19 Antigen Home Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigens from SARS-CoV-2.
Under FDA’s EUA, CareStart™ COVID-19 Antigen Home Test is authorized for non-prescription home use with self-collected anterior nasal (nares) swab samples from individuals aged 14 years or older with symptoms of COVID-19 within the first 7 days of symptom onset. This test is also authorized for non-prescription home use with adult-collected nasal (nares) swab samples from individuals aged 2 years or older with symptoms of COVID-19 within the first 7 days of symptom onset. This test is also authorized for non-prescription home use with self-collected anterior nasal (nares) swab samples from individuals aged 14 years or older, or adult-collected anterior nasal (nares) swab samples from individuals aged 2 years or older, with or without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over three days with at least 24 hours (and no more than 48 hours) between tests.
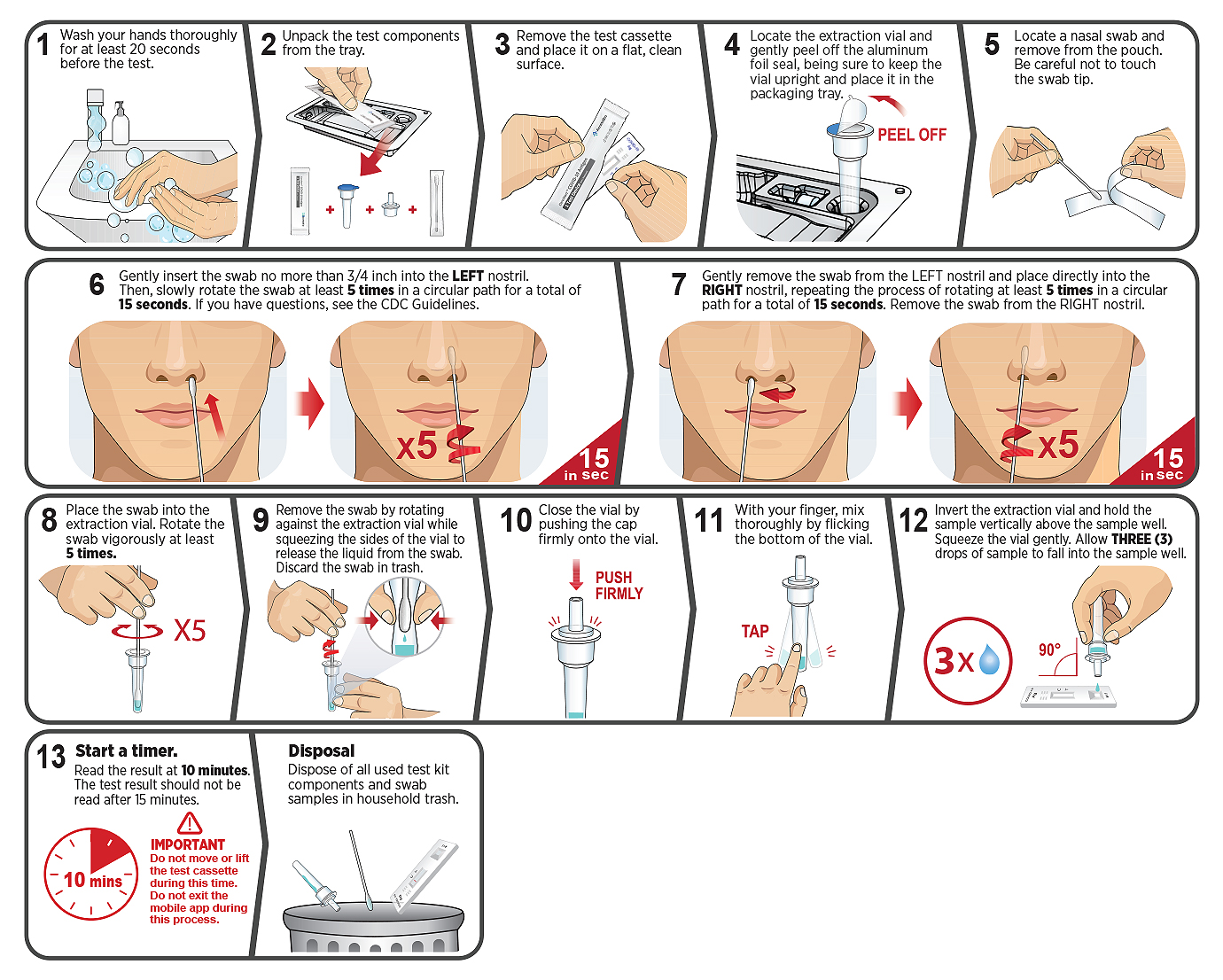
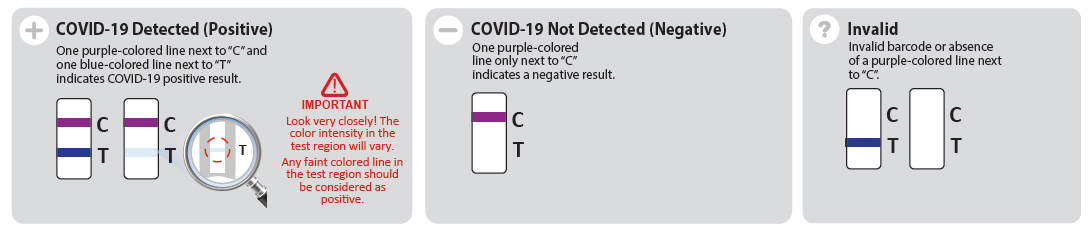
Using the CareStart™ COVID-19 Antigen Home Test

- For use under Emergency Use Authorization Only
- For In Vitro Diagnostic Use Only
Compatible OS Systems:
- iOS13 or newer for Apple iPhone
- Android10 or newer for Android Phone
Disclaimer:
- As of November 22, 2021, CareStart™ COVID-19 Antigen Home Test is authorized to use with individual anterior nasal swab specimens from individuals age 14 years and older (self-collected), or 2 years and older (collected with adult assistance) for non-prescription home use by FDA under the EUA (EUA210314/S002). The authorized labeling has been changed with the new indication (Quick Reference Instructions (QRI) and Fact Sheet for Individuals, Instructions for Use (IFU) for Healthcare Providers (HCP), Fact Sheet for HCP, and kit package); however, CareStart™ COVID-19 Antigen Home Test will continue to be distributed with the labeling previously authorized per EUA210314/S001 on the FDA’s enforcement discretion in efforts to increase the testing accessibility for COVID-19. Since the differences between the CareStart™ COVID-19 Antigen Home Test kit previously authorized (EUA210314/S001) and currently authorized (EUA210314/S002) are limited to the authorized labeling, you may use the CareStart™ COVID-19 Antigen Home Test kit with the new indication for use (described above).